EMAN2 Tomography Workflow Tutorial
- This tutorial is best suited for EMAN2 built after 09/27/2018. Not everything described in the tutorial was functioning yet in the 2.22 release.
This tutorial uses data from EMPIAR: EMPIAR 10064 (only the 4 mixed CTEM tilt series)
- Time estimates for each step are for a well-configured tomography workstation with a high-speed disk, 64+ GB of RAM and 16+ cores.
- The pixel size in the header of the files are incorrect as provided by EMPIAR. The correct Apix value (2.62) should be specified when importing the images.
Prepare input files (~2 minutes)
- Make a new empty folder for the project and 'cd' into that folder
- Make sure any EMAN2 commands you run are executed from within this folder (not any subfolder)
- You may use "Edit Project" from the Project menu to set default values for the project. While not required, it reduces later errors.
- Make sure the workflow mode is set to "TOMO" not "SPR"
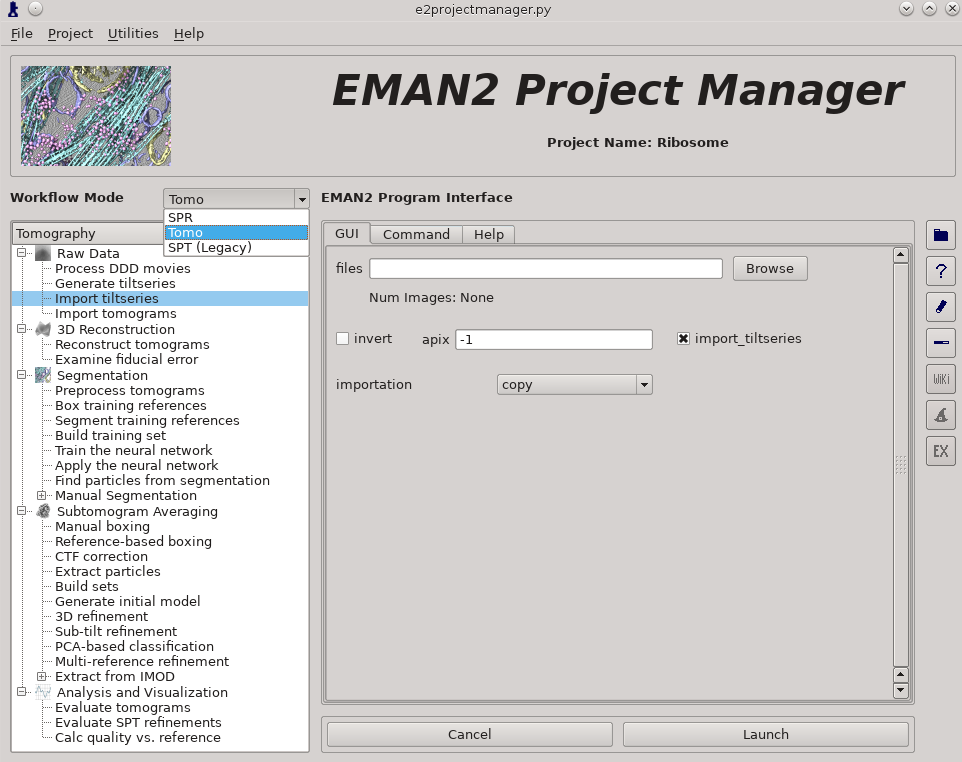
Raw Data -> Import tilt series
Select the files, and make sure importation says copy
In this step you should enter the correct A/pix for your data in the apix box. For EMPIAR10064, this is 2.62. For your own data, you need to know this number. In later steps you should be able to use -1 (default) for apix.
If your tilt series isn't a single stack file, but is many individual images instead, you will need to use Generate tiltseries to build an image stack. This is not necessary for the tutorial data.
Once the options are set, press Launch
It is critical that the filenames for your data not contain any spaces (replace with underscore) or periods (other than the final period used for the file extension). "" (double underscore) is also reserved for describing modified versions of the same file, and should not be used in your original files.
Tiltseries Alignment and Tomogram Reconstruction (20 min)
Alignment of the tilt-series is performed iteratively in conjunction with tomogram reconstruction. Tomograms are not normally reconstructed at full resolution, generally limited to 1k x 1k or 2k x 2k, but the tilt-series are aligned at full resolution. For high resolution subtomogram averaging, the raw tilt-series data is used, based on coordinates from particle picking in the downsampled tomograms. On a typical workstation reconstruction takes about 4-5 minutes per tomogram.
For the tutorial tilt-series:
3D Reconstruction -> Reconstruct Tomograms
check alltiltseries
alternatively you can select one or more tilt series from the tiltseries folder
check correctrot
tltstep = 2
clipz = 64
If you wish to look at the intermediate aligned tilt-series and other files, uncheck notmp
- This is not required for the remaining steps in the tutorial, but can be used to help understand how the tomogram alignment works. This requires significant additional disk space. You may consider doing this for only one tomogram.
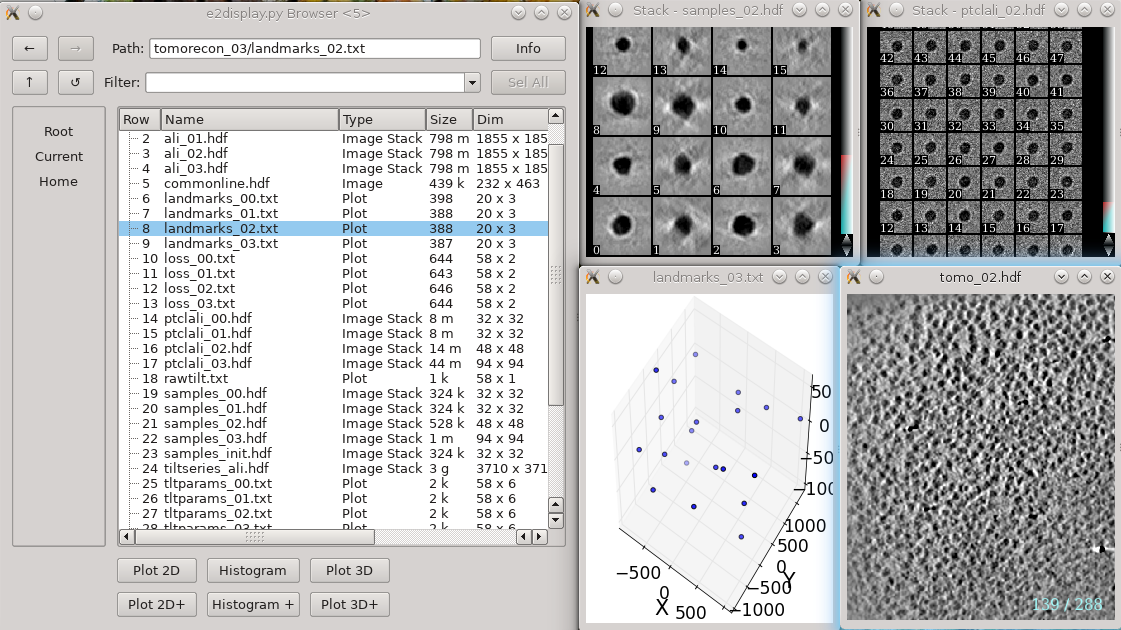
In each tomorecon_XX folder
landmark_0X.txt has the location of the landmarks (which may be fiducials if present) in each iteration
samples_0X.hdf shows the top and side view of those landmarks
ptclali_0X.hdf has the trace of each landmark throughout the tilt series (they should stay at the center of image all the time if the alignment is good)
tomo_0X.hdf is the reconstruction after each iteration
- Launch
When working with your own data:
Either specify the correct tltstep if the tilt series is in order from one extreme to the other, or specify the name of a rawtlt file (as produced by serialem/IMOD).
While the program can automatically compute the orientation of the tilt axis, it is better to fill in the correct value in tltax since there is a handedness ambiguity in the tomogram if determined automatically.
In most cases, the default npk should be fine. If fiducials are present, it is not necessary to adjust this number to match the number of fiducials. The program will use any high contrast areas it finds as potential landmarks.
bytile should normally be selected, as it will normally produce better quality reconstructions at higher speed. If 2k or larger tomograms are created, memory consumption may be high, and you should check the program output for the anticipated RAM usage.
- The graphical interface only permits 1k or 2k reconstruction sizes. In our experience this is normally sufficient for segmentation or particle picking.
When the sample is thin (purified protein, not cells), it is useful to check correctrot to automatically position tomograms flat in ice
It can also be helpful with thin ice to specify a clipz value to generate thinner tomograms (perhaps 64 or 96 for a 1k tomogram).
CTF Estimation (10 min)
For the tutorial tilt-series:
Subtomogram Averaging -> CTF Correction
check alltiltseries
Double check the voltage and cs
- Launch
When working with your own data:
The first two options, dfrange and psrange indicate the defocus and phase shift range to search. They take the format of “start, end, step”, so “2, 5, .1” will search defocus from 2 to 5 um with a step size of 0.1. Units for phase shift is degrees.
For images taken with volta phase plate, we usually have dfrange of “0.2,2,0.1” and psrange of “60,120,2”.
Note that this program is only estimating CTF parameters, taking tilt into account. It is not performing any phase-flipping corrections on whole tomograms. CTF correction is performed later as a per-particle process. This process requires metadata determined during tilt-series alignment, so it cannot be used with tomograms reconstructed using other software packages.
Tomogram annotation (optional)
- Since the tutorial data set is purified ribosomes, this step can be skipped for the tutorial data, and you can move on to template-based particle picking. For cells or other types of complex specimens, tomogram annotation can be used to produce locations of different types of objects.
This section is brief and is only an update to the more detailed tutorial: TomoSeg. Some directory structure and user interfaces have changed in the latest version to match new tomogram workflow as described here:
Segmentation -> Preprocess tomogram
- This step is not always necessary for tomograms reconstructed in EMAN2, but may slightly improve results.
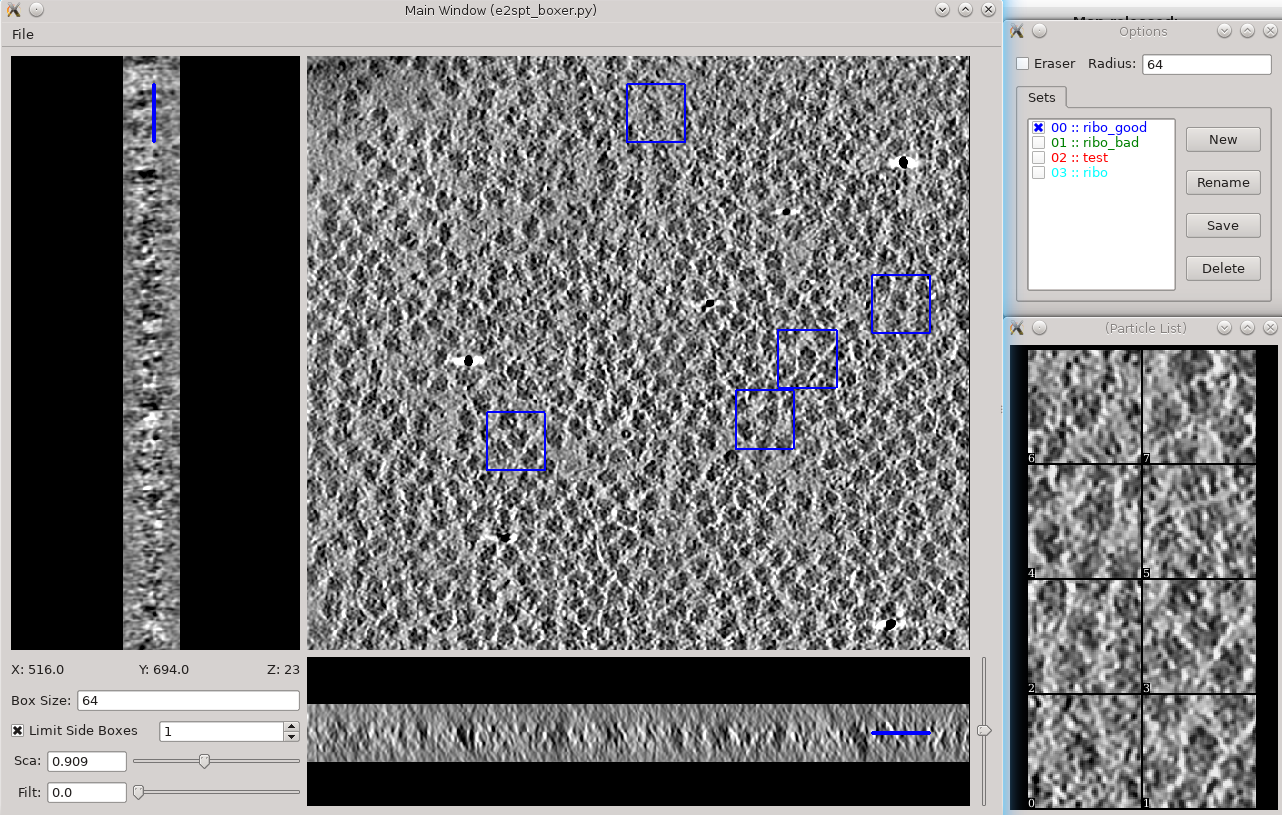
Segmentation -> Box Training References
- This is a newer interface than previously used for this step. Select a few "Good" (regions containing the feature of interest) and "Bad" (regions not containing the feature of interest) boxes.
- "~" and "1" on the keyboard can be used to move along the Z axis.
- The new interface permits different types of features to be identified in a single session and in the same tomogram.
If the different features of interest have very different scale, it is always better to keep the box size at 64, and instead rescale the tomogram. As long as the rescaling is done using EMAN2 utilities, the program will correctly keep track of the geometry relative to the original tomogram & tilt series.
- if you are doing this with the tutorial data, you would only have 2 classes of particles "ribo_good" and "ribo_bad".
When pressing Save all visible particles (box checked next to the class name) will be saved
The rest of the annotation process remain unchanged from the original tutorial, except that now, all trained neural networks and training results are saved in the neuralnets folder, and all segmented maps are in the segmentations folder. You now only specify the label of the output file instead of the full file name.
Segmentation -> Find particles from segmentation to turn segmented maps into particle coordinates.
- Input both the tomogram and its corresponding segmentation, and the particles coordinates will be written into the metadata file.
- Slightly tweaking the threshold parameters may yield better results.
featurename will become the label of particles generated. Those particles can be viewed in the particle picking step and processed in the following protocols.
Particle picking (10-15 min)
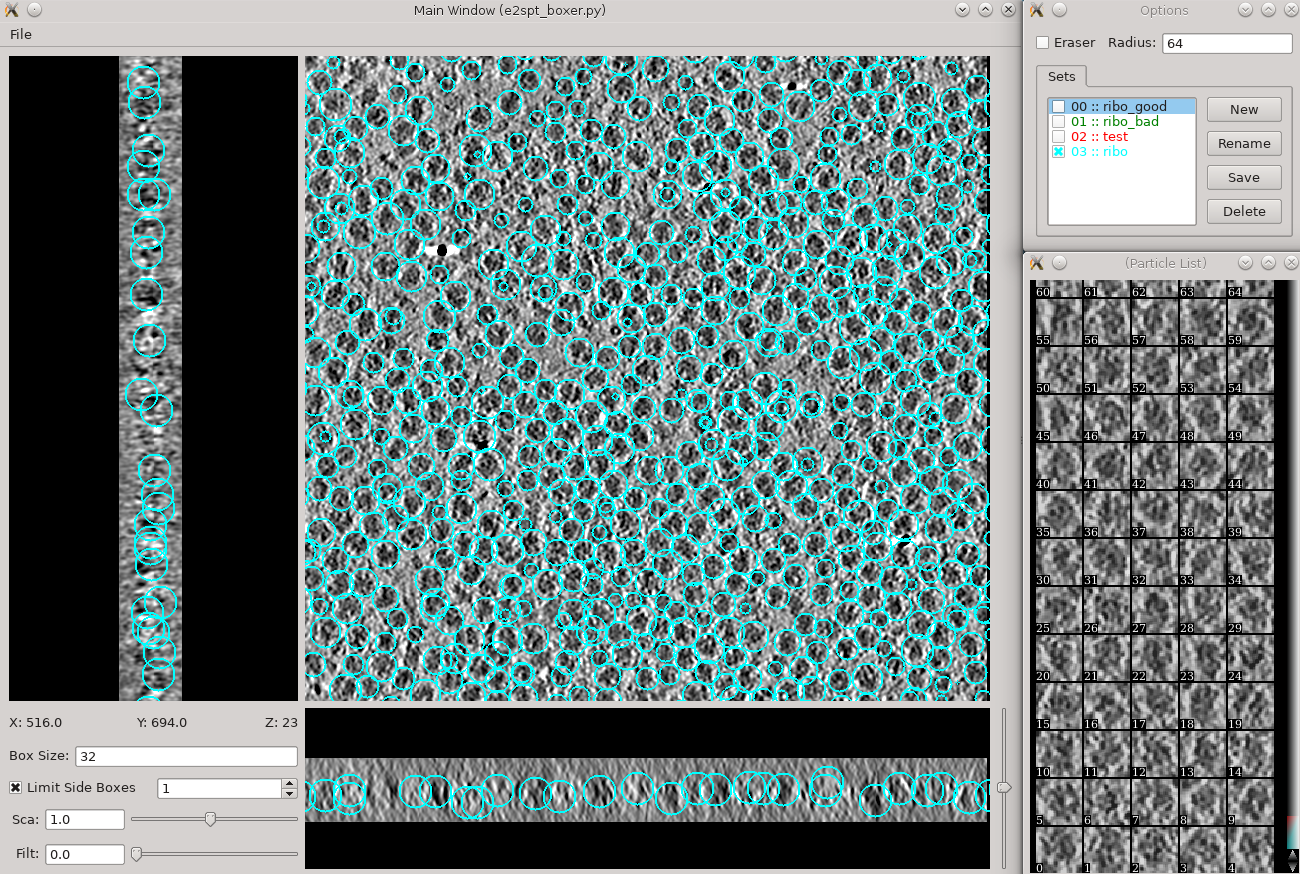
Subtomogram averaging -> Manual boxing Time above is to manually select 30-50 reference particles.
rename the set of boxes to "initribo". This will be used as the label in later stages.
Go through slices along z-axis using ‘~’ and ‘1’ on the keyboard
- left click and drag to place and reposition boxes in any of the 3 views
- Hold down Shift when clicking to delete existing boxes.
- Boxes are shown as circles, which vary in size depending on the Z distance from the center of the particle.
- The interface supports different box types within a single tomogram. Each type has a label. Make sure the label is consistent if selecting the same feature in different tomograms.
- The box size can be set in the main window at the left bottom corner, for the tutorial, use 45 for ribosomes (the unbinned box size is 180).
- If you skipped the tomogram annotation step, we will pick a few particles here to generate an initial model first, and use the initial model as a reference for template matching.
- Select 30-50 particles from a tomogram, then close the boxer window.
- If you have the particle coordinates from tomogram annotation above, you may still wish to do this step to delete any obviously bad particles.
- While you can save 3D particles from the GUI, there is no need to do that here. When you are satisfied with the result, simply close the window.
- You should have ~3000 particles from the 4 tomograms in the dataset.
Particle extraction (2 min)
In this pipeline, the full 1k or 2k tomograms are used only as a reference to identify the location of the objects to be averaged. Now that we have particle locations, the software returns to the original tilt-series, extracts a per-particle tilt-series, and reconstructs each particle in 3-D independently.
For the tutorial tilt-series:
Subtomogram Averaging -> Extract Particles
check alltomograms
set boxsz_unbin to 180.
- If you had the correct size in the previous step this may not be necessary, but it doesn't hurt.
- enter the label you used when picking particles ("initribo" if you followed the instructions above)
- Launch
Subtomogram Averaging -> Build Sets
check allparticles
- Launch
- This will generate particle sets, which are virtual particle stacks that consist of particles with the same label from different tomograms.
For your own data
If the box size is correct when you select particles from the GUI, you can leave boxsz_unbin as -1, so the program will keep that box size (scaled to the original tilt series)
If your particles are deeply buried in other densities, using a bigger padtwod may help, but doing so may significantly increase the memory usage and slow down the process.
With CTF information present, it generally does not hurt to check wiener, which filters the 2D particles by SSNR before reconstructing them in 3D.
Specify a binning factor in shrink to produce downsampled particles if your memory/storage/CPU time is limited, but it will also limit the resolution you can achieve.
Initial model generation (10 - 60 min)
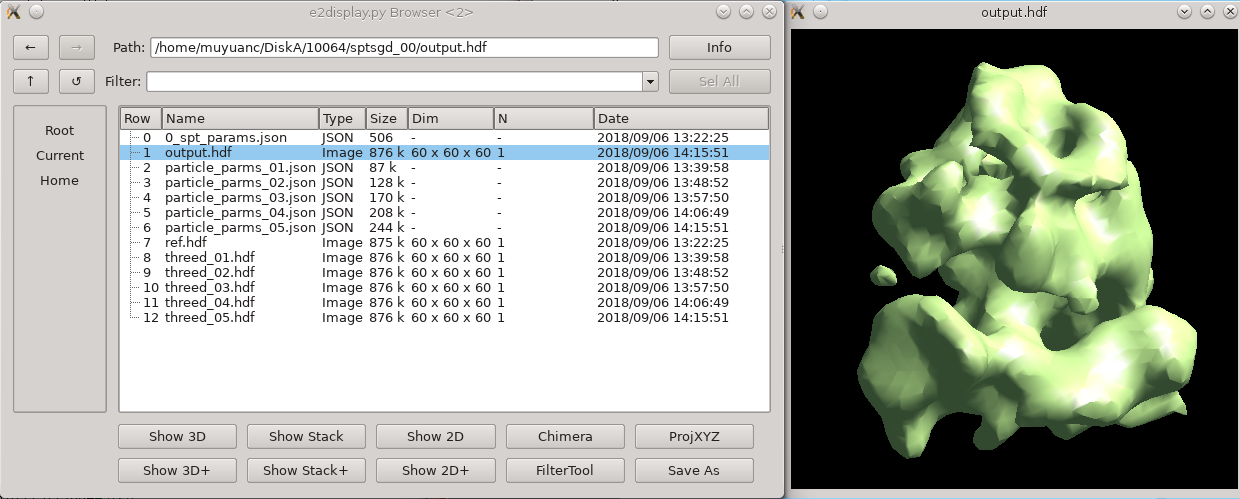
While intuitively it seems like, since the particles are already in 3-D, that the concept of an "initial model" should not be necessary. Unfortunately, due to the missing wedge, and the low resolution of one individual particle (particularly from cells), it is actually critical to make a good starting average, and historically it has been challenging to get a good one, depending on the shape of the molecule. This new procedure based on stochastic gradient descent has proven to be quite robust, but it is difficult for the computer to tell when it has converged sufficiently. For this reason, the default behavior is to run much longer than is normally required, and have a human decide when it's "good enough" and terminate the process. If you use a small shrink value and let it run to completion, it can take some time to run, but this is normally a waste.
For the tutorial tilt-series:
Subtomogram Averaging -> Generate Initial Model
particles should be set to the sets/ribo.lst file you just created (whatever name you used).
set shrink to 2 or 4
- 2 will run slower but will produce a more detailed initial model (not really necessary)
increasing batchsize will use more cores (if you have more than 12), and may cause it to converge to the correct answer in fewer iterations, but each iteration will not become faster.
The default niter of 5 is typically much more than is required
- Launch
You can terminate the job as soon as sptsgd_00/output.hdf looks reasonable. If you display the progress monitor (4th icon on the right side of the project manager), you can easily kill the job when you're happy. Usually this will take about 10 minutes for the tutorial data.
For your own data:
If your particle has known symmetry, specify that EMAN2/Symmetry
The symmetry you specify will not be imposed on the map unless you also check applysym, but the map will be rotationally aligned so the symmetry axes are in the correct direction, which will make it easier to apply symmetry in later steps. We do not generally recommend checking this box in this step.
setting shrink to something in the range of 2-4 will make the runtime faster but, depending on the shape, could potentially cause problems.
- using more than the minimal 30-50 particles is fine. If you have a very large set of selected particles, go ahead and use them all. This will not slow the process down at all, since it's stochastic.
it is critical that the full sampling box size of the extracted particles divided by shrink be divisible by 2. If not, the program will crash.
Template matching (5 min)
In this step, we will use the initial model you just produced as a template for finding all of the ribosomes in all 4 tomograms. If you completed the Tomogram Annotation step above, and have already extracted a full set of 1000+ particles, then you can skip this step, as we already have all of the particles.
Subtomogram Averaging -> Reference Based Boxing
browse to select tomograms. Select all 4 tomograms.
set reference to the output.hdf file you produced in the previous step.
set label to "ribo"
set nptcl to 800 (the maximum number of particles per tomogram)
- Launch
when this finishes, you can use the same Manual Boxing tool you used before to look at the particles which were selected. You may wish to manually remove any bad particles it selected. For the tutorial data set or other tomograms of purified protein, this process should work pretty well. For cells you might wish to use the Tomogram Annotation method above.
- note that this process stores 3-D particle locations in the appropriate info/* files, but does not extract particles from the micrographs
Particle extraction (~1 hour)
Again, if you already did Tomogram Annotation above, this step isn't necessary. It is only required if you just did Template Matching.
Since this involves several thousand particles instead of 30-50, it will take quite a lot longer to run. The actual time will depend partially on the speed of your storage.
For the tutorial tilt-series:
Subtomogram Averaging -> Extract Particles
check alltomograms
set boxsz_unbin to 180.
set label to "ribo"
- Launch
Subtomogram Averaging -> Build Sets
check allparticles
- Launch
- This will generate particle sets, which are virtual particle stacks that consist of particles with the same label from different tomograms.
Subtomogram refinement
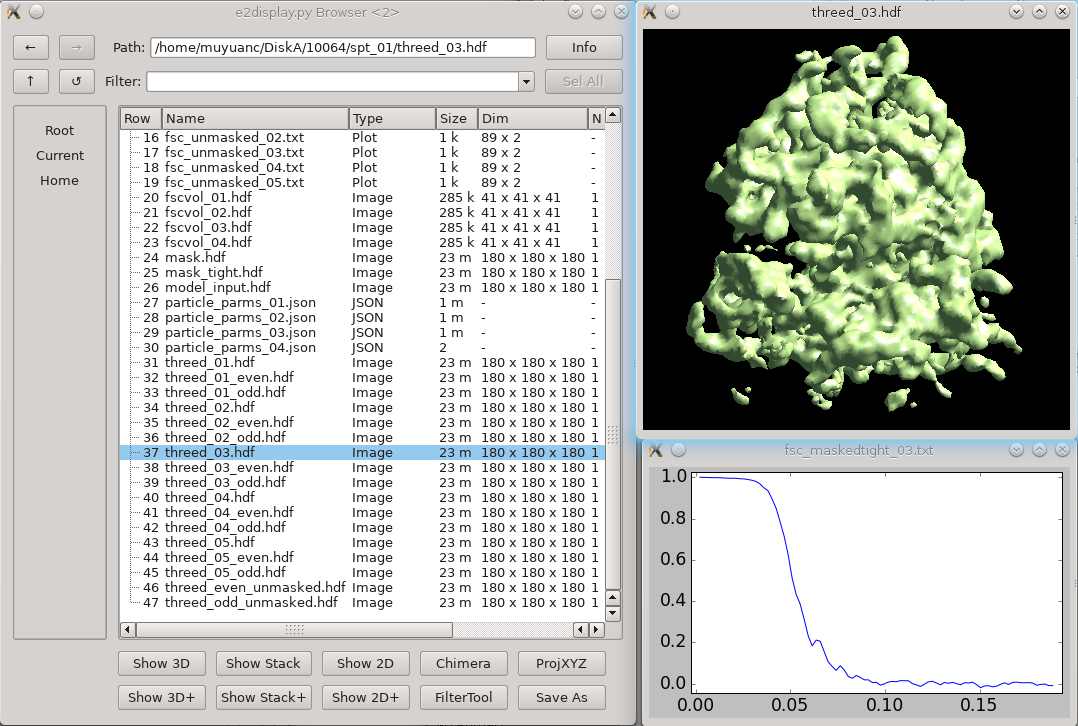
Click 3D refinement from the left panel, and input both the particle set and the initial model generated from the last step as a reference. If there is a symmetry of the protein, make sure it is aligned to the symmetry axis before specifying the correct symmetry. If you are willing to split the even/odd set of particles and do a “gold-standard” refinement, specify a resolution number (usually 30-50) in goldstandard, so information beyond that resolution will be randomized independently in the reference for even and odd set. While it is good to have a reasonable mass for the molecular weight of protein (in kDa) and tarres for the target resolution, leaving them as default usually does not hurt. If you have a known structure factor in a .txt file, (you can compute it from a known structure via e2proc3d.py), specify it in setsf. localfilter will filter the averaged map by local resolution, which is especially useful when looking at things in cells where parts of proteins can be very flexible. This is almost always good to check when you want to push toward high resoluion. pkeep controls the fraction of particles that go into the final average. If you know there are many bad particles in the dataset, setting it to be a smaller number may help. Enter the number of threads you want to use in the thread option. Finally, click Launch and wait. For this dataset, it can take a few hours on a decent workstation. The results can be seen in the spt_XX folder. In the folder, threed_XX.hdf files are the main output map after each iteration, and fsc_masked/unmasked/masktight_XX.txt files are the FSC curves between even/odd half set under different masking. You should be able to get to 12-15Å resolution (cutoff 0.143) at this step using this dataset.
Subtilt refinement
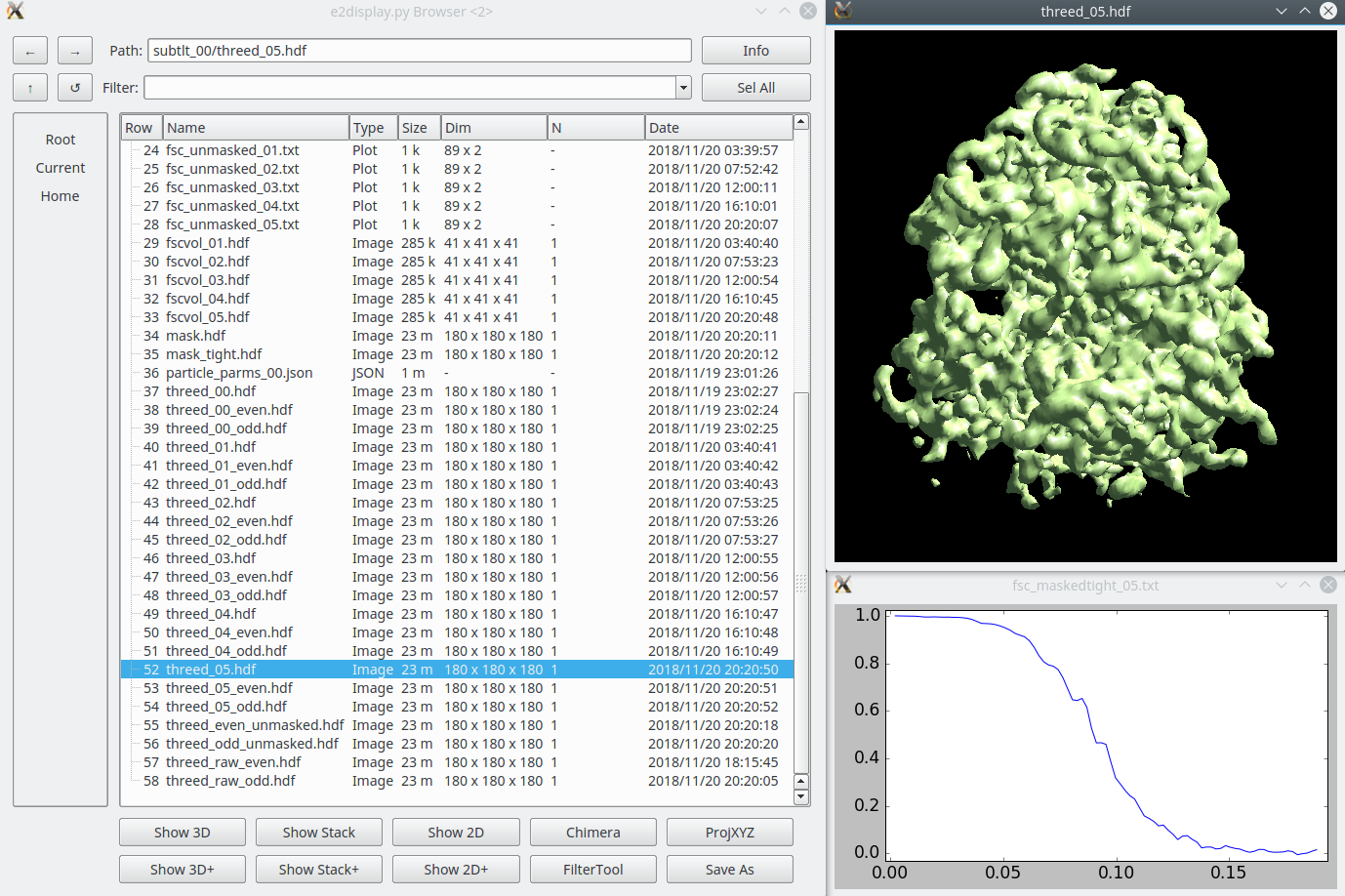
Once the subtomogram refinement finishes, check the final map and FSC curves. In this dataset, you should be able to achieve a resolution of 13-15Å. Now we can refine the orientation of each individual subtilt, i.e. 2D particles from raw tilt series that are reconstructed into to the 3D particles, and push the resolution of the averaged map.
Click Sub-tilt refinement, choose the folder of the last subtomogram refinement and launch the program. You will need to specify the path to the spt_XX directory containing the last completed subtomogram refinement (typically just “spt_00” for example). Additionally, specify the iter you want to use as a starting point for sub-tilt refinement. If “-1” is specified, the program will attempt to locate the last complete iteration.
The default parameters should be generally fine for this dataset, though you may need to alter the parallel and threads options to use the number of CPU threads available on your computer. The niters value corresponds to the number of iterations of sub-tilt refinement you wish to perform. keep controls the fraction of particles that goes into the final map. If you are certain that tilt images beyond a certain angle (for example, 45 degrees) are radiation damaged, you can put 45 in maxalt, and specify a larger keep number. Otherwise, just use keep 0.5, so the program will judge the quality of subtilt images by their correlation to the averaged map and exclude worst 50% 2D particles.
Tomogram evaluation
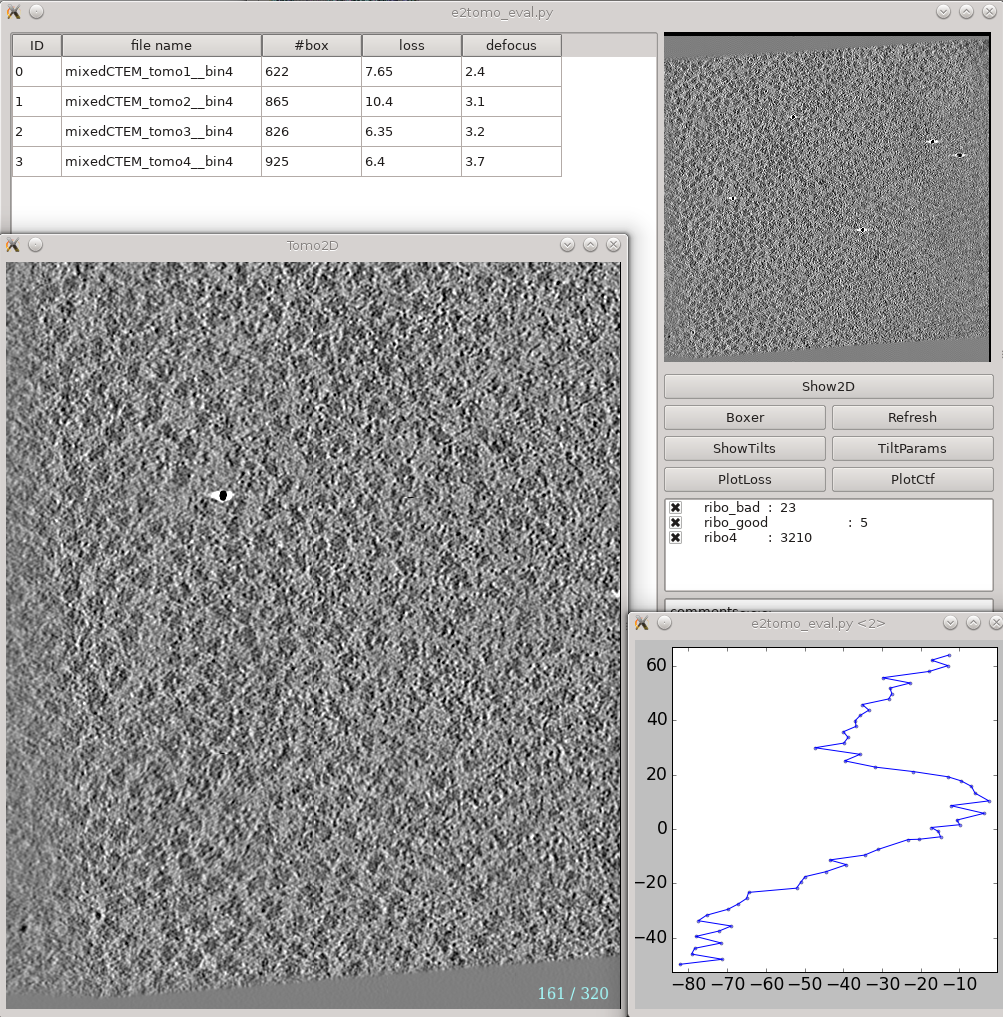
This is a tool that helps you visualize your tomograms with their corresponding metadata, and launch other programs from it. It can be found via Analysis and visualization -> Evaluate tomograms. This can be used at any point of the workflow after tomogram reconstruction.
On the left is a list of tomograms in the project. Clicking the header of each column will sort the table by that attribute. #box is the number of boxes in the tomogram, loss is the average fiducial error in nm, and defocus is the average defocus of the tilt series. Do not be scared by large loss values here. Although the relative value of different tomograms (aligned with the same parameters) in the same project are correlated with tiltseries quality, the exact value here is not as meaningful. You can still get a subnanometer resolution subtomogram average from tilt series with a loss larger than 5 nm.
On the right, the image on the top shows the center slice of the tomogram. The Show2D button shows the selected tomogram in slices, ShowTilts shows the corresponding raw tilt series, and Boxer calls the 3D boxer. PlotLoss will plot the fiducial error per each tilt, and PlotCtf plot the defocus and phase shift at the center of each tilt image. Tiltparams is a bit more complicated. It plots a point list with 6 columns and a number of rows corresponding to the images in the selected tilt series. These are the alignment parameters for the tilt series. The columns represent tilt ID, translation along x and y-axis, tilt angle around y, x and z-axis correspondingly. You can adjust X Col and Y Col in the plot control panel (middle click the plot) to change the display. The first panel below the buttons are the types of particle and their numbers in the dataset. Check and uncheck the boxes will affect the number displayed in #box column on the left. The last box is reserved for comments for each tomogram. You can fill in any comments you have for the selected tomogram and it will be saved with other metadata of the tomogram for future references.
Refinement evaluation
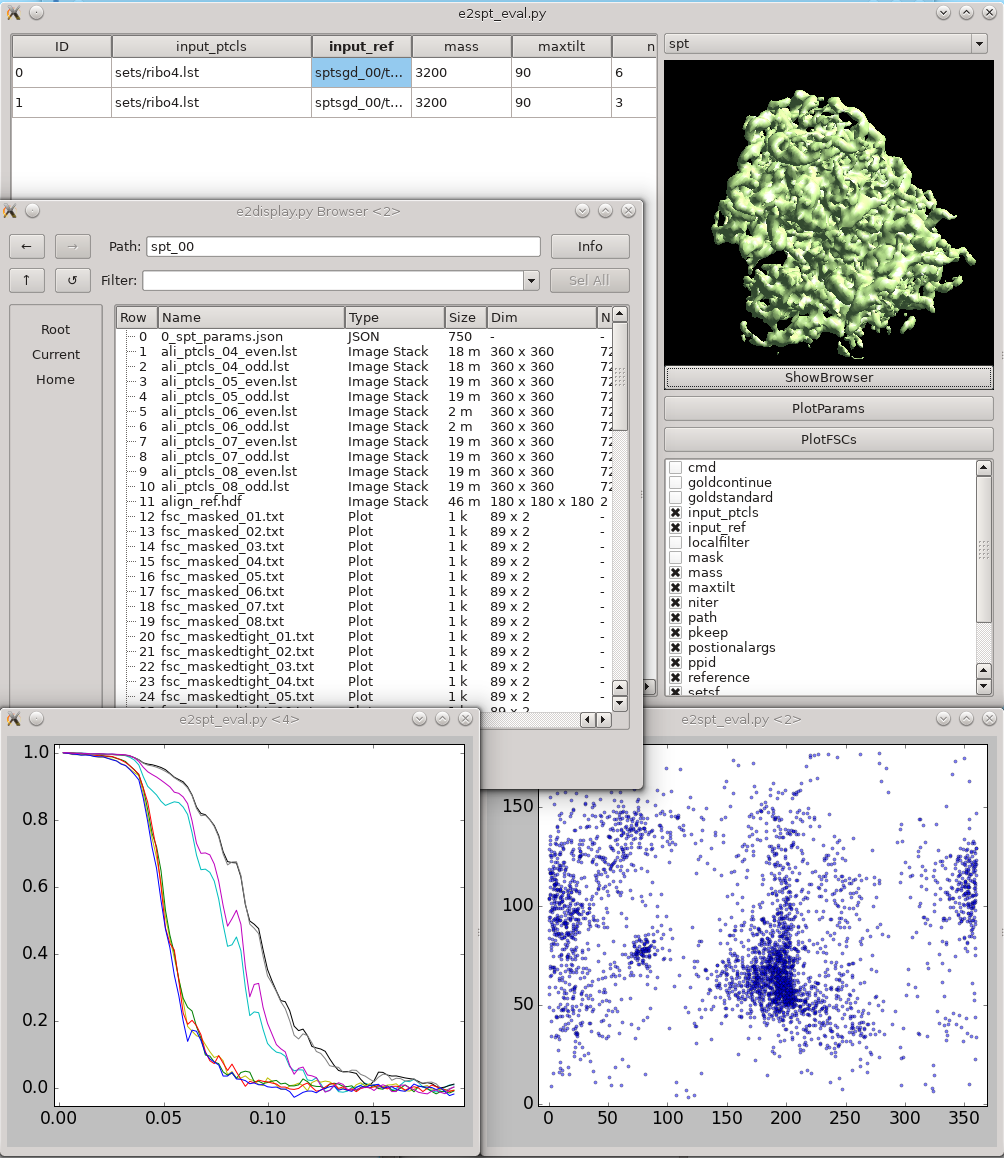
This tool helps visualize and compare results from multiple subtomogram refinement runs. Launch it from Analysis and visualization -> Evaluate SPT refinement. In the GUI, you can look at all spt_XX and sptsgd_XX folders and compare their options and resulting maps. Switch between folder type using the menu at top right. Click the header of a column to sort the table by its content. Uncheck items in the list at bottom-right to hide corresponding columns. Clicking ShowBrowser will bring up the e2display.py browser in the folder of the selected row. PlotParams will plot the Euler angle distribution and other alignment parameters. The 8 columns in the plot are three Euler angles (az, alt, phi), translation in x,y,z, alignment score, and missing wedge coverage score. PlotFSCs will plot the FSC curve under tight mask from each iteration.
